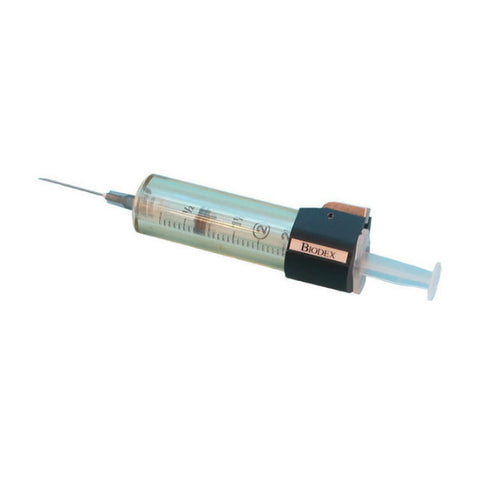
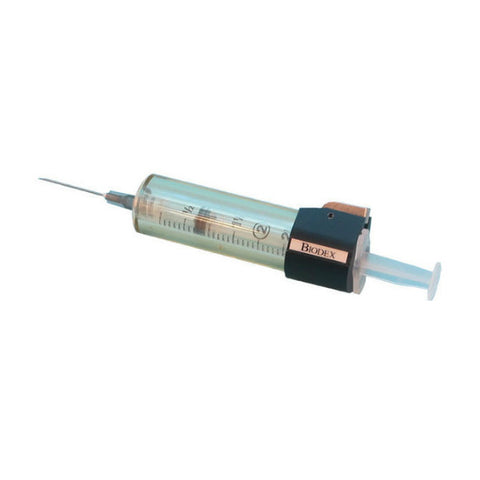
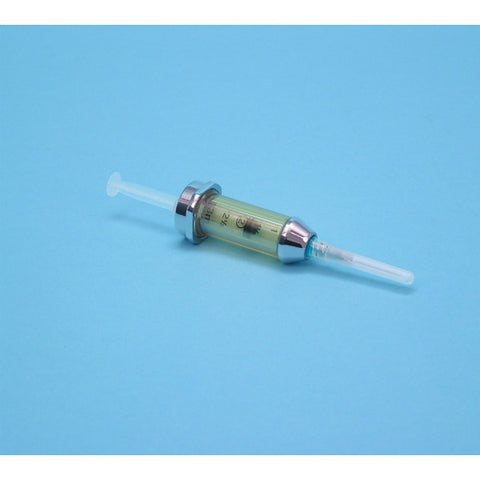
- Designed for optimum radiation protection during elution/compounding/dispensing activities
- Patent pending grip mechanism improves handling
- Accommodates all 10 mL vials including NetSpot ®
- Compatible with all commercially available Ge68/Ga68 generators
- 95% Tungsten
- Shielding features and designs incorporate USP<797/825> considerations
- Height 3.9”
- Shielding 0.89”
- Weight 12.8 lb
- Product Code: G10V
SWE Design Considerations USP <797/825>
“US federal and state radiation regulatory authorities require limiting radiation exposure to personnel who handle radiopharmaceuticals, which necessitates special provisions for radiation protection”
“Radiation exposure to personnel decreases as a function of shielding materials.
Therefore, handlers of radiopharmaceuticals may use various shielding materials
(e.g., lead, tungsten) in various configurations.”
SWE Shields are composed of 95% Tungsten to drastically decrease exposure to
personnel while enhancing ergonomics and handling
“…it is necessary to balance aseptic handling practices (patient safety) with
radiation protection practices (worker safety).”
SWE Shields provide an aseptic milieu for radiopharmaceuticals during production,
transportation or administration
“…the most important factor for maintaining sterility is the avoidance of touch
contamination”
SWE Syringe and Vial Shields contain patent pending inserts designed to mitigate touch contamination within the PEC (Primary Engineering Control) as well as during dose calibration and administration
“Disinfection of the vial septum with sterile 70% isopropyl alcohol (IPA) must be
performed prior to needle puncture.”
SWE shields are easily sanitized with 70% IPA or commonly used sanitizing agents
“If the vial shield top is then closed or the vial septum otherwise covered with a piece
of radiation shielding, the septum must be re-disinfected with sterile 70% IPA prior
to another needle puncture. Some vial shields are constructed such that the vial septum is recessed and difficult to access.”
SWE Vial shields are designed with recessed septum opening to mitigate potential contamination of the septum, while allowing for efficient/effective sanitation of the vial septum.